Forecasting how long it will take to get a new product to market is difficult at best, but when developing products for highly regulated markets such as pharmaceuticals and medical devices strict testing regime can add additional risk and uncertainty. For example, US Food and Drug Administration (FDA) mandates multi-phase approval process for validation of safety, efficacy, and efficiency of new drug and vaccines. The possible impact of testing regimes on the cost and time required for successful product development can be modeled using Probabilistic Branching in combination with Monte Carlo simulations.
Risks and Uncertainties in Pharmaceutical Industry
The regulatory regimes that govern the development and marketing of medical products (pharmaceuticals, devices, etc) add an additional level of complexity, but also paradoxically a level of certainty to these projects. The complexity comes from add regulatory requirements which include stringent performance and quality measures. These same measures also create very distinct decision points in the project that branch in two specific directions: Pass or Fail. Pass moves the project into the next phase, which consumes additional cost, time and resources. Fail will end the project with some exceptions. While this would seem to be a poor outcome, in many cases it can be viewed as a success as early failures limits the impact of additional sunk costs that accrue and would be lost when products fail later tests or during market introduction. So in many cases, companies may want new product development projects to fail early or not at all.
Probabilistic Branching in Testing Regimes
The best method to model the impact of testing regimes on product development is probabilistic branching. Each test in the development process is represented by a branch of Pass or Fail. The probability that a particular test will Pass or Fail can be based on historical data, expert judgement, a specific known characteristics of the product in question. If the test Fails, this branch is cancelled. If it passes, the branch is successful and it successor activities are executed as planned. Due to the multiple branches and many opportunities for failures, the chance that a new product will make it to market can be exceedingly small; calculating the expected values for cost and duration can only be accomplished using Monte Carlo simulations.
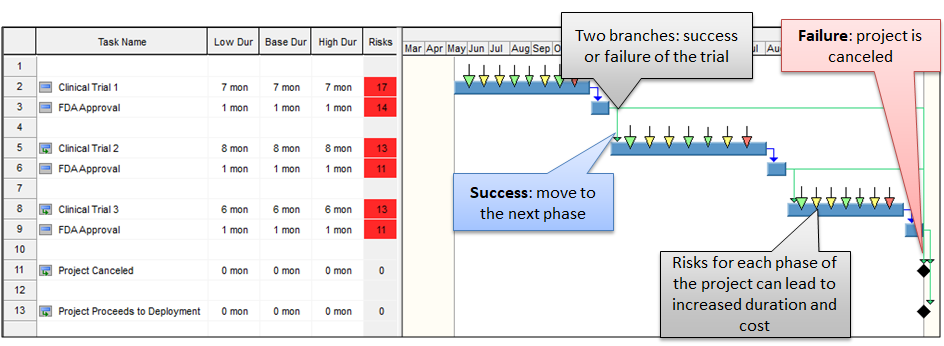
How to implement Probabilistic Branching
If you are currently using Monte Carlo simulations to model project cost and schedule, simulations with probabilistic branching shares many of the same processes. First is to identify and assess your project risks with probability and impact. This information is combined with your project schedule (preferably resource loaded) by assigning the project risks and uncertainties to activities and resources. At each testing point, a probabilistic branch is created using two successors, one for the probability of success and one with a probability of failure. Run a Monte Carlo simulation which will generate two results. The first is that it will rank and prioritize the risks for mitigation based on their impact on costs and schedule. The second, is that it will generate statistical distributions that will provide histograms and cumulative probabilities for project costs and schedule. This is an iterative process that can be performed at the beginning of each phase or as new information becomes available.
If you would like more information on probabilistic branching or project risk analysis our book: Project Risk Analysis Made Ridiculously Simple provides an easy to follow guide to project risk analysis.




